A recent recall of Costco’s Kirkland Signature Severe Cold & Flu Plus Congestion medication has raised concerns among consumers. With contamination identified as the primary reason, this event underscores the critical importance of vigilance in pharmaceutical quality control. As one of the leading retailers, Costco’s proactive approach to addressing the issue highlights its commitment to consumer safety.
The Recall Details
Costco’s recall involves its over-the-counter Kirkland Signature Severe Cold & Flu Plus Congestion medicine, specifically Item #1729556, with the lot code P140082. The product was sold between October 30 and November 30, 2024, at select Midwest and Southeast locations. The recall was initiated after discovering potential foreign material contamination. In its official notice, Costco and the product’s manufacturer, LNK International Inc., urged customers to discontinue use immediately and return the product for a full refund.
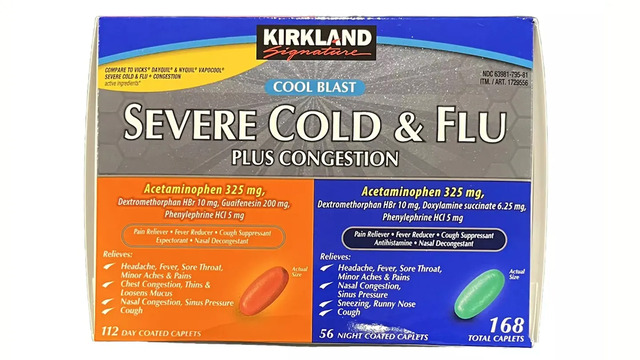
Affected consumers were directed to contact LNK International Inc. for assistance via their helpline (1-800-426-9391) or email ([email protected]). The company’s transparency and the quick dissemination of information are vital steps in maintaining trust.
Video:
Implications for Consumer Health
While no specific health incidents related to this contamination have been reported, the recall brings to light the risks of foreign material contamination in pharmaceutical products. Contaminants could compromise the effectiveness of the medication and, in worst-case scenarios, pose serious health risks. The recall also highlights the need for consumers to stay vigilant, checking lot numbers on their purchases and monitoring updates from retailers and manufacturers.
A Second Recall in a Month
This is the second time in less than a month that Costco’s cold and flu remedies have been under scrutiny. In January 2025, 8,640 boxes of Kirkland Severe Cold & Flu Plus Congestion Day and Night packs were recalled following an FDA finding that oral phenylephrine, a key ingredient, was ineffective as a nasal decongestant. According to Dr. Marc Siegel, Fox News’ senior medical analyst, phenylephrine’s effectiveness diminishes in oral forms unless administered at doses that could cause adverse cardiovascular effects.
This double recall may lead consumers to question the reliability of store-brand medications and highlights the critical need for stringent quality control measures within the pharmaceutical industry.

Consumer Reactions and Concerns
Consumers have expressed mixed reactions to the recall. While some appreciate Costco’s transparency and quick action, others worry about the frequency of recalls and the potential risks of consuming contaminated or ineffective products. The trust consumers place in brands like Costco is integral to their purchasing decisions, making it essential for the retailer to reinforce its commitment to safety and quality.
The Role of Industry Standards
Recalls like these emphasize the importance of adhering to stringent pharmaceutical manufacturing and quality standards. The FDA plays a pivotal role in ensuring that over-the-counter products meet efficacy and safety benchmarks. However, manufacturers must also take proactive measures to monitor production and maintain high standards to avoid lapses like contamination.
Retailers, on their part, bear the responsibility of ensuring the products they sell meet consumer expectations. Costco’s swift response to the issue demonstrates its dedication to addressing concerns, but ongoing vigilance is essential to prevent such incidents in the future.
Steps Taken by Costco

In response to the recall, Costco has taken immediate steps to address the issue, including:
- Issuing a public recall notice to inform consumers.
- Ensuring affected customers are offered full refunds.
- Coordinating with the manufacturer to investigate the root cause of contamination and implement preventive measures.
These actions reinforce Costco’s commitment to prioritizing consumer safety, even at the potential expense of temporary brand reputation concerns.
Expert Insights on Product Safety
Dr. Marc Siegel emphasizes the importance of consumer education regarding medication safety. He notes that while certain ingredients, like phenylephrine, may be deemed ineffective, the greater concern arises when potentially harmful contaminants are introduced. Dr. Siegel advises consumers to remain informed about recalls and consult healthcare professionals when in doubt.
Consumer Advice
For consumers, the recall serves as a reminder of the importance of vigilance. Key tips include:
- Check Lot Numbers: Always verify product lot numbers against recall notices.
- Monitor Retailer Websites: Stay updated on product recalls through trusted sources like Costco’s website.
- Dispose of Recalled Products: Safely discard any affected items or return them for refunds as instructed.
- Consult Professionals: If adverse effects are suspected, seek medical advice promptly.
Conclusion
The recall of Costco’s cold remedy highlights the complexities of ensuring pharmaceutical safety and the importance of proactive measures to address potential risks. While the incident may raise questions, Costco’s transparent handling of the situation showcases its dedication to consumer well-being. As recalls prompt both industry and consumers to remain vigilant, they also serve as opportunities to strengthen trust and improve safety standards.
Ultimately, the collective effort of manufacturers, retailers, and regulatory bodies is vital in upholding the integrity of the products we rely on. Consumers, too, play a crucial role in staying informed and advocating for transparency, ensuring a safer future for all.



